1. Period for Abstract Submission
Deadline: 22nd November, 2024 (Japan Standard Time) by 12 noon.
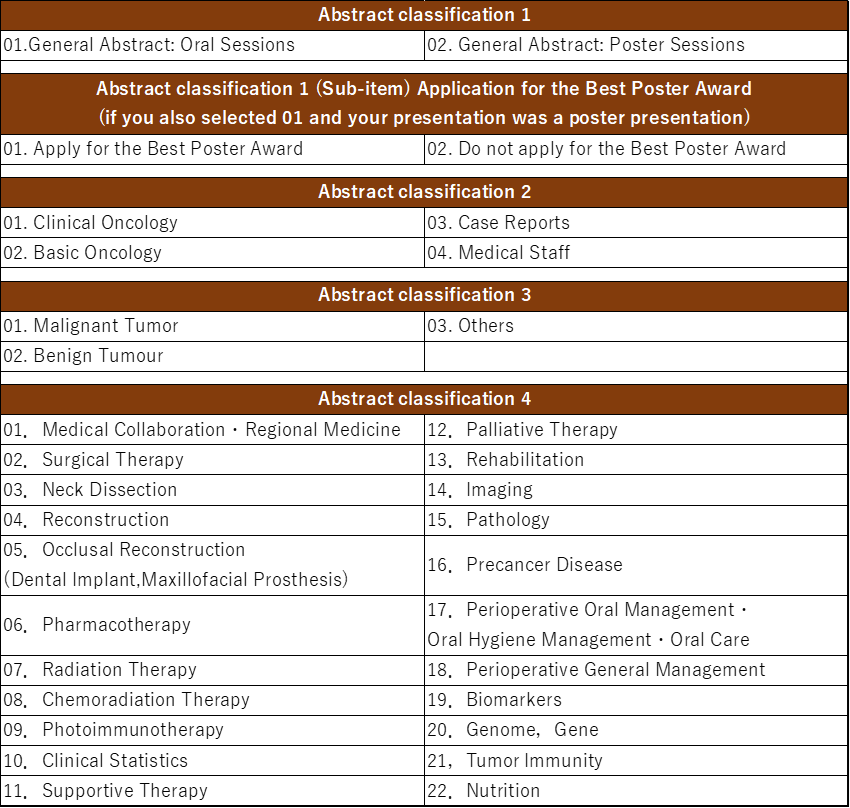
2. Presentation Type & Abstract Classification
Presentation Type
(1)Oral Presentation
(2)Poster Presentation
All presenters are required to take their presentations on-site regardless of presentation type.
Please be advised that the conference organizer will make the final decision on the types of presentations regardless of your preference.
*Case reports are also eligible for the Best Poster Award. When submitting your entry, please be sure to clearly state what you are trying to promote, such as rare cases or new treatments.
3. Notification of Acceptance
The notification of acceptance will be sent to email address registered on the online portal system late December
4. Submission
Title: Maximum of 120 half-width characters, including subtitle.
Abstract: Maximum of 1200 half-width characters.
*The abstracts will be online published in the program book
5. Abstract Submission System
(1) About the browsers
Internet Explorer, Microsoft Edge, Netscape, Safari, FireFox, and Google Chrome are the browsers that can be used with the UMIN online abstract submission system. Please do not use any other browsers.
(2) Conflict of Interest
Clinical research based on industrial-academic cooperation not only returns to society the results obtained by researchers executing their scientific and ethical responsibilities (public benefit), but also sometimes generates money, standing and concessions, etc., that are acquired by individual researchers in association with such industrial-academic cooperation (personal gains). The situation where these two types of interests occur at the same time in regard to an individual researcher is known as “conflict of interest”. The JSOO started the submission and disclosure of matters of conflict of interest by officers of the JSOO, presenters. When you submit your abstract, please disclosure whether or not you have any conflict of interest (COI) disclosure.
Conflict of interest disclosure is required for clinical research presentations, and is applicable to the first speaker's own abstract from one year prior to the abstract submission date to the time of the presentation, and to any business or for-profit organization related to the content of the presentation. Co-authors are not required. Basic research using cultured cells or laboratory animals is not eligible.
Oral presentation: If there is any COI, the oral presenter should use the disclosure slide to present COI after the title slide, in order to clarify the names of companies and/or associations in question. If there is no COI, the presenter should indicate ‘I/We have no financial relationships to disclose as such on the sample slide.
Poster presentation: If there is any COI, the poster presenter should disclose the names of companies and/or associations in question at the bottom area of the poster. If there is no COI, the presenter should indicate ‘I/We have no financial relationships to disclose.’ as such on the sample slide.
①With regard to a position as an officer or an advisor (including consultant, etc.) of the Companies, etc., if annual value of remuneration received from a single Company, etc. is 1 million yen or more, a declaration must be made.
②With regard to share ownership, if the annual profit (the sum of dividends and profit on sale) from shares of a single Company, etc. during a one-year period is 1 million yen or more (in the case of stock options, if the latent profit is 1 million yen or more), or if the total proportion of shares owned in the Company, etc. is 5% or more, a declaration must be made.
③With regard to patent royalties or transfer gains received from a single Company or for-profit organization, if the total annual value is 1 million yen or more in total, a declaration must be made.
④With regard to lecture fees, honoraria, or other fees paid by a single Company or for-profit organization for the time or labor of a researcher engaged for conference attendance (as a lecturer, chairperson, ad hoc advisor, etc.), if the total annual value is 500,000 yen or more in total, a declaration must be made.
⑤With regard to manuscript fees for a pamphlet, etc. paid by a single Company or for-profit organization, if the total annual value is 500,000 yen or more in total, a declaration must be made.
⑥With regard to research funds (joint research funds, contract research funds, clinical trial funds, etc.) provided directly under contracts by a single Company or for-profit organization, or by a non-profit organization funded by the Company, etc., if the total annual value is 2 million yen or more, a declaration must be made. With regard to scholarship (incentive) endowments provided directly from a single Company or for-profit organization, or research grants provided directly from a private academic support organization, if the total annual value is 2 million yen or more, a declaration must be made.
⑦With regard to remuneration (travel expenses, gifts, etc., that are not directly related to research), if the annual remuneration received from a single Company, etc. is 50,000 yen or more, a declaration must be made.
Please refer to the sample below to prepare the COI disclosure slide.
●COI Sample
●COI Sample (No COI)
(3)Ethical Procedure for abstract submission (under construction)
6. Abstract Submission via System
【Contact/Secretariat office】
Plando JAPAN Co. Ltd
2 Chome-3-6 Shibadaimon, Minato City, Tokyo 105-0012
e-mail: jsoo43@nta.co.jp
